Water Testing Data Descriptions
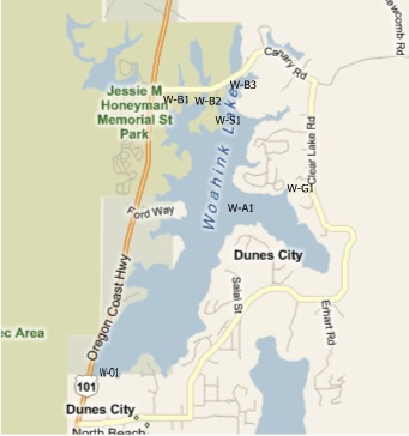
Woahink Lake
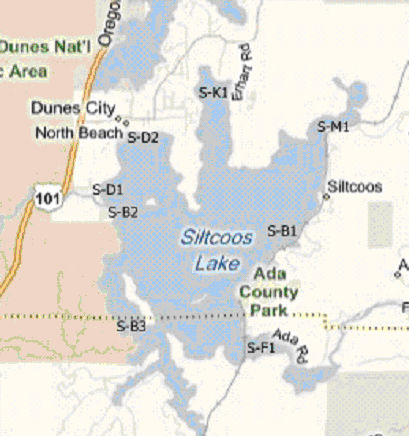
Siltcoos Lake
Explanation of Lake Water Testing Data Base
EXPLANATION OF WATER TESTING DATA
Some of the data appearing on our website is self-explanatory, such as the name of the water body, the date and time of collection and the air and water temperatures. Other data may not be so clear. Consequently, we offer the following information for clarity:
Event Number: This is an internal assignment of a number so the water testers can accurately track the results and enter them into the spreadsheet when they are returned from the laboratories.
Location Code: These are the sites at which the samples are taken. They have been predetermined and all testing and sampling occurs at the same sites each time. Included with this explanation are maps showing the various locations where samples and tests may be taken.
Depth in Meters: This is the depth at which the particular water sample is taken.
TECH: Are the initials of the water testers that were present when the sample was taken or test was run.
Clarity: The measurement for clarity is done by what is called a Secchi disk reading. The larger the number, the more clear the water.
E Coli MPN: E-Coli MPN is a method of determining the presence of Eschirchia coli based the most probably number (MPN). These test results are used to determine if further E-Coli testing should be done. The acceptable level of E. coli is determined by risk analysis based on statistics to protect human health. Drinking water should have no E. coli after treatment. E. coli levels at designated swimming beaches should not exceed 88 per 100 milliliter (mL) in any one sample, or exceed a three-sample geometric mean average over a 60-day period of 47/100mL. Recreational waters that are not designated beaches should not have more than 406 E. coli/100mL in any one sample, or more than 126/100mL in a 60-day, three-sample geometric mean average. Occasional higher numbers are not unusual, particularly after storm events and where urban or agricultural runoff occurs. These levels are generally not considered unsafe unless investigation indicates the source to be sewage.
Chl-A ug/L: This test identifies the micrograms per liter of Chlorophyll-a in a test sample. Chlorophyll a is a specific form of chlorophyll used in oxygenic photosynthesis. It absorbs most energy from of violet-blue and orange-red light. It also reflects green/yellow light, and as such contributes to the observed green color of most plants. This photosynthetic pigment is essential for photosynthesis in eukaryotes, cyanobacteria and prochlorophytes. Chlorophyll is a measure of all green pigments whether they are active (alive) or inactive (dead). Chlorophyll a is a measure of the portion of the pigment that is still active; that is, the portion that was still actively respiring and photosynthesizing at the time of sampling. The amount of algae found in a lake greatly affects the lake’s physical, chemical, and biological makeup. Algae produce oxygen during daylight hours but use up oxygen during the night and again when they die and decay. Decomposition of algae also causes the release of nutrients to the lake, which may allow more algae to grow. Their processes of photosynthesis and respiration cause changes in lake pH, and the presence of algae in the water column is the main factor affecting Secchi disk readings. Algae, of course, also can cause aesthetic problems in a lake; a green “scum,” and rotting scent are common problems associated with high algae concentrations. The higher the reading, the more algae is in the water.
Nitrate: In freshwater or estuarine systems close to land, nitrate can reach high levels that can potentially cause the death of fish. While nitrate is much less toxic than ammonia, levels over 30 ppm (______ ug/L) of nitrate can inhibit growth, impair the immune system and cause stress in some aquatic species. However, in light of inherent problems with past protocols on acute nitrate toxicity experiments, the extent of nitrate toxicity has been the subject of recent debate.
In most cases of excess nitrate concentrations in aquatic systems, the primary source is surface runoff from agricultural or landscaped areas that have received excess nitrate fertilizer. This is called eutrophication and can lead to algae blooms. As well as leading to water anoxia and dead zones, these blooms may cause other changes to ecosystem function, favoring some groups of organisms over others. As a consequence, as nitrate forms a component of total dissolved solids, they are widely used as an indicator of water quality.
Ttl Phos ug/L: This test identifies the micrograms per liter of Phosphorus in a test sample. Phosphorus is usually present in natural water as phosphates (orthophospates, polyphosphates, and organically bound phosphates). Phosphorus is a plant nutrient needed for growth and a fundamental element in the metabolic reactions of plants and animals (hence its use in fertilizers). Sources of phosphorus include human and animal wastes (i.e., sewage), industrial wastes, soil erosion, and fertilizers. Excess phosphorus causes extensive algal growth called “blooms,” which are a classic symptom of cultural eutrophication and lead to decreased oxygen levels in lake water.
Silicates: Silicon is known to be present in all living organisms. This element occurs in the form of hydrated amorphous silica, referred to as opal, and is required for the production of structural materials in single-celled organisms through to higher plants and animals. For many life forms, silicon can even be considered to be an essential element. The assessment of the effects of soluble silicate emissions must be reviewed in this context. To understand the effects of increased silicate concentrations, one must understand the biological function of silicate in water ecosystems, which is complex. In water systems, algae (and aquatic plants) are responsible for primary production: the transformation of inorganic nutrients and carbon dioxide into organic biomass. The algae biomass (density) is determined by several, potentially limiting, factors, such as temperature, light intensity and nutrient concentrations. All algae, such as green and bluegreen algae, require phosphorus and nitrogen as nutrients, but only diatoms (a type of algae) also require silicate for growth. Dunes City has not historically tested for Silicates.
Total Nit ug/L: This test identifies the micrograms per liter of Nitrogen in a test sample.
Nutrients, such as nitrogen and phosphorus, are essential for plant and animal growth and nourishment, but the overabundance of certain nutrients in water can cause a number of adverse health and ecological effects. Nitrogen, in the forms of nitrate, nitrite, or ammonium, is a nutrient needed for plant growth. About 78% of the air that we breathe is composed of nitrogen gas, and in some areas of the United States, particularly the northeast, certain forms of nitrogen are commonly deposited in acid rain. Excess nitrogen can cause overstimulation of growth of aquatic plants and algae. Excessive growth of these organisms, in turn, can clog water intakes, use up dissolved oxygen as they decompose, and block light to deeper waters. Lake and reservoir eutrophication can occur, which produces unsightly scums of algae on the water surface, can occasionally result in fish kills, and can even “kill” a lake by depriving it of oxygen. The respiration efficiency of fish and aquatic invertebrates can occur, leading to a decrease in animal and plant diversity, and affects our use of the water for fishing, swimming, and boating. A high level is 1.80 mg/L (______ ug/L)
Turb NTU: Is a measure of Turbidity in Nephelometeric Turbidity Units. Turbidity is the cloudiness or haziness of the water caused by large numbers of individual particles that are generally invisible to the naked eye, similar to smoke in air. The measurement of turbidity is a key test of water quality. Turbidity in open water may be caused by growth of phytoplankton. Human activities that disturb land, such as construction, mining and agriculture, can lead to high sediment levels entering water bodies during rain storms due to storm water runoff. Areas prone to high bank erosion rates as well as urbanized areas also contribute large amounts of turbidity to nearby waters, through stormwater pollution from paved surfaces such as roads, bridges and parking lots. Governments have set standards on the allowable turbidity in drinking water. In the United States, systems that use conventional or direct filtration methods turbidity cannot be higher than 1.0 nephelometric turbidity units (NTU) at the plant outlet and all samples for turbidity must be less than or equal to 0.3 NTU for at least 95 percent of the samples in any month. Systems that use filtration other than the conventional or direct filtration must follow state limits, which must include turbidity at no time exceeding 5 NTU.
pH: Is a numeric scale used to specify the acidity or basicity (alkalinity) of the lake water. Water with a pH less than 7 are acidic and solutions with a pH greater than 7 are basic. Pure water is neutral, being neither an acid nor a base. Contrary to popular belief, the pH value can be less than 0 or greater than 14 for very strong acids and bases respectively.
DO ppm: Is the measure of dissolved oxygen in parts per million. Fish, invertebrates, plants, and aerobic bacteria all require oxygen for respiration. Much of the dissolved oxygen in water comes from the atmosphere. After dissolving at the surface, oxygen is distributed by current and turbulence. Algae and rooted aquatic plants also deliver oxygen to water through photosynthesis. The main factor contributing to changes in dissolved oxygen levels is the build-up of organic wastes. Decay of organic wastes consumes oxygen and is often concentrated in summer, when aquatic animals require more oxygen to support higher metabolisms. Depletions in dissolved oxygen can cause major shifts in the kinds of aquatic organisms found in water bodies. Temperature, pressure, and salinity affect the dissolved oxygen capacity of water. The ratio of the dissolved oxygen content (ppm) to the potential capacity (ppm) gives the percent saturation, which is an indicator of water quality. Most fish do well when the dissolved oxygen is five parts per million or higher. When the dissolved oxygen is less than five ppm they become uncomfortable.
Btm Depth meters: How far, in meters, is the bottom of the lake from the surface. Water testers historically haven’t done this calculation.
Conduct. uS/cm: Conductivity (or specific conductance) of an electrolyte solution is a measure of its ability to conduct electricity. In many cases, conductivity is linked directly to the total dissolved solids (T.D.S.). High quality deionized water has a conductivity of about 5.5 µS/m, typical drinking water in the range of 5-50 mS/m, while sea water about 5 S/m[2] (i.e., sea water’s conductivity is one million times higher than that of deionized water). The values in µS/cm are lower than those in µS/m by a factor of 100 (i.e., 1 µS/cm = 100 µS/m).
